We may not have the course you’re looking for. If you enquire or give us a call on 01344203999 and speak to our training experts, we may still be able to help with your training requirements.
We ensure quality, budget-alignment, and timely delivery by our expert instructors.

Interested to know what the ISO 17025 Handbook consists of? This comprehensive guide provides laboratories valuable insights and practical strategies for improving their competence by ISO 17025 standards. Packed with easy-to-understand content, this handbook is a valuable resource for laboratories aiming to enhance their testing and calibration processes, especially when following the standards outlined in the ISO 17020 Guide for improved efficiency and accuracy.
ISO 17025 is widely adopted globally, with thousands of testing and calibration laboratories accredited. This blog will act as an ISO 17025 Handbook, providing a comprehensive and practical guide to implementing and maintaining quality management systems in testing. Read more to learn more!
Table of Contents
1) Background of ISO 17025
2) Key concepts and terminology
3) Understanding ISO 17025 requirements
4) Benefits of an ISO 17025 Handbook
5) Conclusion
Background of ISO 17025
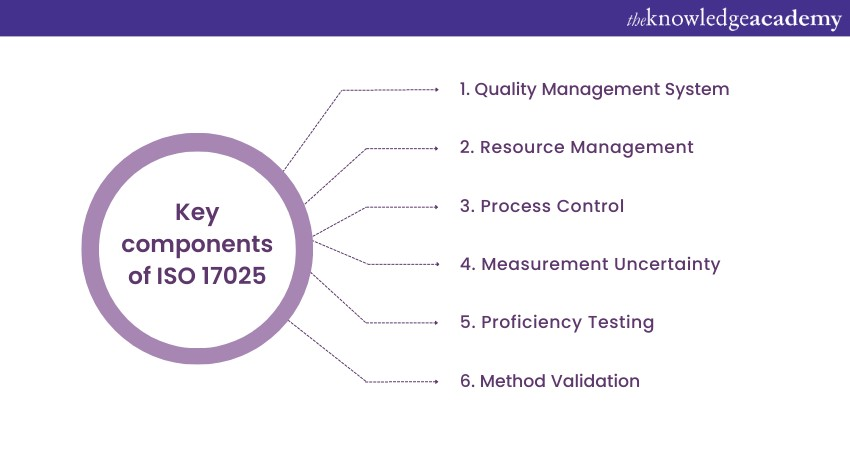
ISO 17025 is an internationally recognised standard for testing and calibration laboratories. This standard was first published in 1999 and has since been revised to adapt to industry practices and technological advancements. The most recent major revision occurred in 2017, emphasising a process-based approach and risk-based thinking. ISO 17025 covers various aspects of laboratory operations, including quality management systems, resource management, and process control.
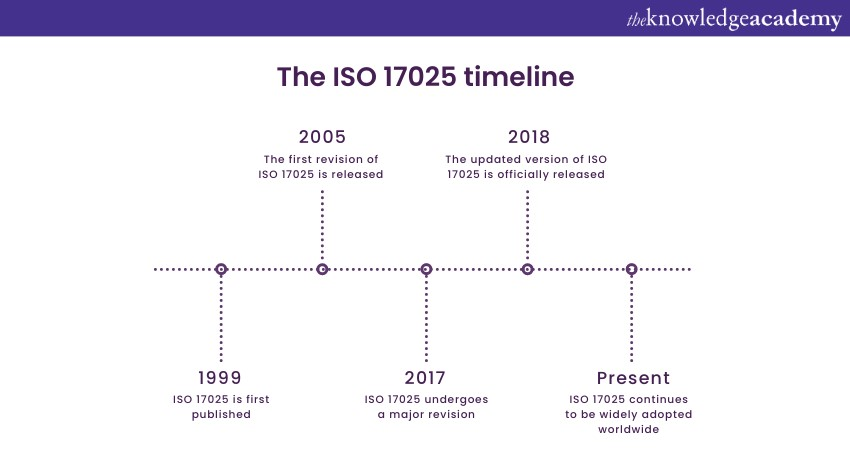
Laboratories must establish documented procedures and processes to effectively control and monitor their operations. Measurement uncertainty and proficiency testing are vital requirements. Accreditation bodies assess compliance with ISO 17025. Understanding the Benefits of ISO 17025 Accreditation is essential, as it enhances laboratory credibility, improves operational efficiency, boosts customer confidence, and ensures accurate results. Implementing ISO 17025 standard improves functions, boosts customer confidence, and ensures accurate results. Labs must understand and follow ISO 17025 for a robust quality management system and continuous improvement.
Master the basics of ISO 17025 implementation by registering in our ISO 17025 Lead Implementer Training Join now!
Key concepts and terminology
In ISO 17025, several important terms play a significant role in establishing laboratory competence and ensuring the quality of test results. Let's explore their definitions and their relevance:
Accreditation: Accreditation is the formal recognition given by an accreditation body to a laboratory, indicating that it has demonstrated competence and compliance with the requirements of ISO 17025. Accreditation verifies that the laboratory meets international standards and follows best practices, enhancing its credibility and confidence in its test results.
Traceability: Traceability refers to the ability to relate test results to internationally recognised measurement standards through an unbroken chain of calibrations. It ensures that the results obtained by the laboratory can be linked to recognised references, increasing the accuracy and reliability of measurements.
Proficiency testing: Proficiency testing involves participating in external programs where the laboratory's performance is assessed by comparing its results to those of other participating laboratories. It helps validate the laboratory's competence, identifies areas for improvement, and ensures consistent and accurate testing.
Measurement uncertainty: Measurement uncertainty represents the doubt or range within which the true value of a measured quantity lies. It quantifies the level of confidence in a test result, accounting for various factors that introduce variability. Estimating and controlling measurement uncertainty is crucial to ensure reliable and valid test results.
Understand planning and implementation of the auditing process with our ISO 17025 Lead Auditor Training Sign up now!
Understanding ISO 17025 requirements
To develop a QMS based on ISO 17025, consider these key requirements:
a) Documented policies: Establish and maintain documented policies and procedures outlining quality objectives and processes.
b) Management responsibility: Demonstrate leadership, ensure QMS effectiveness, and foster a culture of quality.
c) Competence and training: Ensure personnel are competent through training and competency assessments.
d) Equipment and calibration: Establish procedures for equipment selection, verification, and calibration.
e) Document and record control: Implement procedures for document and record management to maintain conformity.
f) Internal audits: Conduct regular internal audits to assess QMS effectiveness and identify areas for improvement.
g) Nonconformity management: Address nonconformities through documented procedures, corrective actions, and preventive measures.

Benefits of an ISO 17025 Handbook
Implementing an ISO 17025 Handbook offers unique and exceptional benefits for laboratories. Some benefits include:
a) The handbook provides clear guidelines and standardised practices for laboratories to follow, ensuring uniformity in testing and calibration processes.
b) It helps them improve their understanding of ISO 17025 requirements, implement best practices, and strengthen their entire quality management system.
c) The handbook serves as a valuable resource to enhance the quality of test results.
d) It helps them develop the necessary ISO 17025 Documentation, processes, and procedures required for accreditation.
e) It encourages laboratories to regularly review and update their processes, identify areas for enhancement, and implement corrective actions.
f) Implementing ISO 17025 practices outlined in the handbook can increase customer satisfaction.
g) The ISO 17025 Handbook aligns laboratories with international standards and best practices.
Boost your auditing skills with our ISO 17025 Internal Auditor Training. Sign up now for certified competence and accurate results!
Conclusion
We hope this blog helps you understand the importance of the ISO 17025 Handbook. By following the guidelines and recommendations presented in this handbook, laboratories can establish robust quality management systems, optimise resources, and ensure accurate and reliable test results. ISO 17025 serves as a valuable tool for laboratories striving to excel in the field of testing and calibration.
Gain a comprehensive understanding of the key terms and normative references of ISO 17025. Register for our ISO 17025 Training today to learn more!
Upcoming Health & Safety Resources Batches & Dates
Date
 ISO 17025 Foundation
ISO 17025 Foundation
Mon 18th Aug 2025
Mon 24th Nov 2025






 Top Rated Course
Top Rated Course


 If you wish to make any changes to your course, please
If you wish to make any changes to your course, please


