We may not have the course you’re looking for. If you enquire or give us a call on + 1-866 272 8822 and speak to our training experts, we may still be able to help with your training requirements.
Training Outcomes Within Your Budget!
We ensure quality, budget-alignment, and timely delivery by our expert instructors.

If you are involved in testing and calibrating processes or seeking accreditation for your laboratory, understanding and implementing the ISO 17025 Checklist is essential. But surprisingly, many organisations are still unaware of this certification's benefits.
According to the National Center of Biotechnology Information, only 4% of European organisations use ISO 17025 for medical laboratory accreditation. So, to enjoy the benefits of ISO 17025 certification, you must fulfil the standard’s checklist. Don't know how? Read this blog to learn about the ISO 17025 Checklist and discover the steps laboratories must take to ensure quality and success in testing and calibration.
Table of contents
1) What is the ISO 17025 Checklist?
2) How to prepare for ISO 17025 Certification?
a) Familiarise with the ISO 17025 Standard
b) Conduct internal audits
c) Analyse the audit results
d) Carefully document
e) Apply for ISO 17025 Certification
3) Importance of ISO 17025 Accreditation
4) Conclusion
What is the ISO 17025 Checklist?
The ISO 17025 Checklist is vital for laboratories seeking accreditation and striving for excellence in testing and calibration. This comprehensive checklist encompasses a wide range of requirements that laboratories must meet to demonstrate their competence, impartiality, and consistent operation. Preparing the list and fulfilling the requirements assists organisations in obtaining the certification successfully.
It includes five key sections: general, structural, resource, process, and management system requirements. So, before obtaining the certification, the laboratories require preparing and fulfilling the Checklist.

How to prepare for ISO 17025 Certification?
ISO 17025 Certification is a significant achievement for testing and calibration laboratories. It demonstrates that a laboratory meets international standards for quality and competence. However, before obtaining the certification, laboratories must fulfil the requirements on the preparation list for ISO 17025 Certification. The following steps can guide you through the process:
1) Familiarise with the ISO 17025 Standard
Start by thoroughly understanding the requirements outlined in the ISO 17025 Standard. Study the standard’s clauses, guidelines, and recommendations to understand the expectations and criteria for accreditation clearly. Also, familiarise yourself with the management and technical requirements and the documentation and quality control procedures.
2) Conduct internal audits
Internal audits are essential to evaluate your laboratory’s compliance with the ISO 17025 Standard. Therefore, conducting systematic assessments of your processes, procedures, and documentation is crucial to identify any gaps or areas that require improvement. Laboratories also require engaging qualified auditors or establishing an internal audit team to perform these assessments objectively and effectively.
3) Analyse the audit results
Once the internal audits are complete, analyse the audit findings and identify areas of non-compliance or improvement opportunities. Prioritise the issues based on their impact and develop action plans to address them effectively. Further, engage relevant stakeholders to ensure a collaborative approach to rectify the identified gaps.
Unlock the potential for excellence in laboratory quality management. Register for our ISO 17025 Training now!
4) Carefully document
Accurate and comprehensive documentation is crucial to obtain the ISO 17025 certification. Ensure that all relevant procedures, policies, and records are documented clearly and conform to the standard’s requirements. Additionally, document control, including version control, review cycles, and accessibility to demonstrate effective management of laboratory documentation.
5) Apply for ISO 17025 Certification
Lastly, it is time to apply for ISO 17025 Certification. Select an accredited certification body specialising in laboratory accreditation and submit your application. The certification body will conduct an assessment to verify your compliance with the ISO 17025 Standard.
The certification body will review your documentation during the assessment, perform on-site inspections, and interview staff members. They will assess your laboratory’s competence, quality control procedures, equipment calibration, personnel training, and other relevant aspects. Successful completion of the assessment can get you the ISO 17025 Certification.
Importance of ISO 17025 accreditation
ISO 17025 accreditation holds significant importance for testing and calibration laboratories. But how is it significant? What benefits can it provide? Why is the certification essential? Let's explore the key reasons why ISO 17025 accreditation is essential:
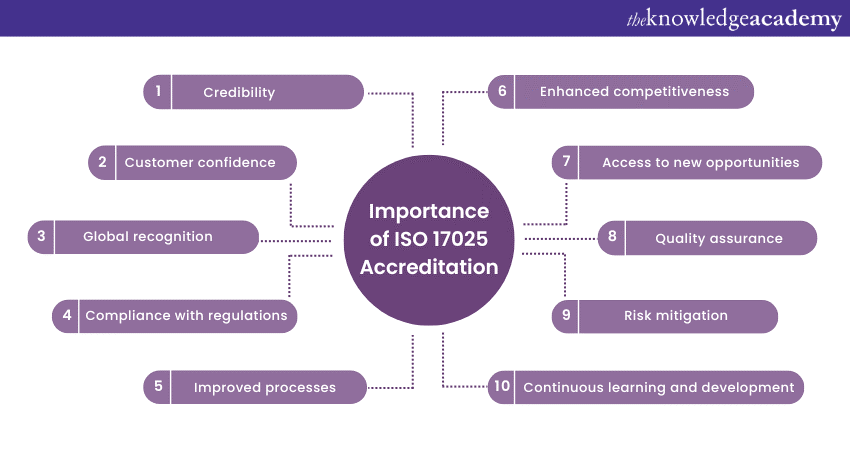
a) ISO 17025 Accreditation enhances the credibility of a laboratory by demonstrating its compliance with international standards for quality and competence.
b) It helps assure customers that the laboratory’s testing and calibration processes meet rigorous quality requirements, instilling confidence in the accuracy and reliability of results.
c) 17025 is internationally recognised; thus, it facilitates acceptance of test and calibration results across borders and fosters global partnerships.
d) Accreditation ensures the laboratory’s compliance with relevant industry regulations, standards, and guidelines. It further helps reduce the risk of non-compliance and potential legal issues.
e) Implementing ISO 17025 requirements promotes continuous improvement within the laboratory, leading to enhanced operational efficiency and effectiveness.
f) The accreditation sets laboratories apart from competitors, demonstrating their commitment to quality and providing a competitive advantage in the marketplace.
g) It also opens doors to new business opportunities, such as government contracts, collaborations, and partnerships with organisations that require accredited laboratories.
h) Laboratories get to implement robust quality management systems, leading to consistent and reliable testing and calibration results.
i) The laboratories can easily identify and mitigate risks associated with testing and calibration, ensuring the accuracy and reliability of results.
j) Accreditation also encourages laboratories to stay updated with the latest advancements in their field, fostering a culture of professional development.
Learn how to address risks and opportunities at workplace with our ISO 17025 Lead Implementer course.
Conclusion
If you aspire to achieve ISO 17025 accreditation for your laboratory, use the ISO 17025 Checklist as your roadmap to success. Ensure compliance with the standard’s requirements, establish a robust quality management system, and consistently strive for excellence in your operations.
Take your laboratory to new heights of excellence with our ISO 17025 Foundation course.







 Top Rated Course
Top Rated Course



 If you wish to make any changes to your course, please
If you wish to make any changes to your course, please


