We may not have the course you’re looking for. If you enquire or give us a call on +44 1344 203 999 and speak to our training experts, we may still be able to help with your training requirements.
Training Outcomes Within Your Budget!
We ensure quality, budget-alignment, and timely delivery by our expert instructors.

Picture a scenario: You are an experienced Auditor and are considering a new role in the thriving medical device industry. Moreover, you're intrigued by the potential of becoming an ISO 13485 Auditor, but one question lingers: "What can I anticipate in terms of salary?" That's where our blog on ISO 13485 Auditor Salary can help you.
In addition, do you often wonder how geographical location, experience, and industry demand shape the earning potential for these specialised roles? Our blog covers it all.
Table of Contents
1) Significance of ISO 13485 Auditing
2) ISO 13485 Auditor Salary Range
a) Experience
b) Qualifications and Certifications
c) Geographic Location
d) Organisation Size
e) Additional Skills and Expertise
3) Conclusion
Significance of ISO 13485 Auditing
ISO 13485 Auditing plays a vital role in ensuring the best standards of quality and regulatory compliance within the medical device industry. Let's delve into the key reasons why ISO 13485 Auditing holds immense importance:
1) Compliance With International Standards: ISO 13485 auditors assess whether organisations comply with this standard by conducting audits. Compliance is crucial to demonstrate a commitment to quality, regulatory compliance, and patient safety.
2) Enhanced Product Quality and Safety: ISO 13485 auditing helps identify areas where the quality management system may fall short, leading to non-conformities. By addressing these non-conformities and suggesting corrective actions, auditors contribute to improving the overall quality and safety of medical devices.
3) Risk Mitigation: Through their assessments, they can pinpoint weaknesses, vulnerabilities, and gaps that may pose risks to product quality or patient safety. By addressing these risks, auditors help organisations mitigate potential hazards and ensure the efficacy and reliability of medical devices.
4) Continuous Improvement: ISO 13485 auditing promotes a culture of continuous improvement within organisations. Through the audit process, auditors provide valuable feedback and recommendations for enhancing the quality management system.
5) Global Market Access: Many countries and regulatory bodies require compliance with ISO 13485 as a prerequisite for market access. By obtaining ISO 13485 certification, organisations can demonstrate conformity with international standards and gain a competitive edge in the global marketplace.
6) Customer Confidence and Trust: The ISO 13485 certification serves as tangible evidence that an organisation has implemented robust quality management systems and is committed to meeting customer expectations and regulatory obligations.
ISO 13485 Auditor Salary Range
The annual ISO 13485 Auditor Salary in the United Kingdom ranges between £30,000 to £60,000. Below is a table highlighting their income potential based on experience levels:
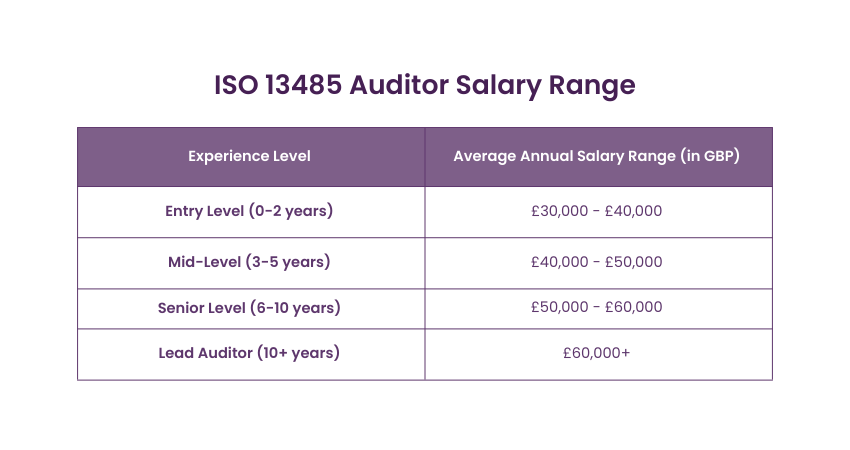
Source: Glassdoor
However, it is important to note that the salary range can vary significantly based on the factors. Here are the key factors that contribute to the salary range of ISO 13485 Auditors:
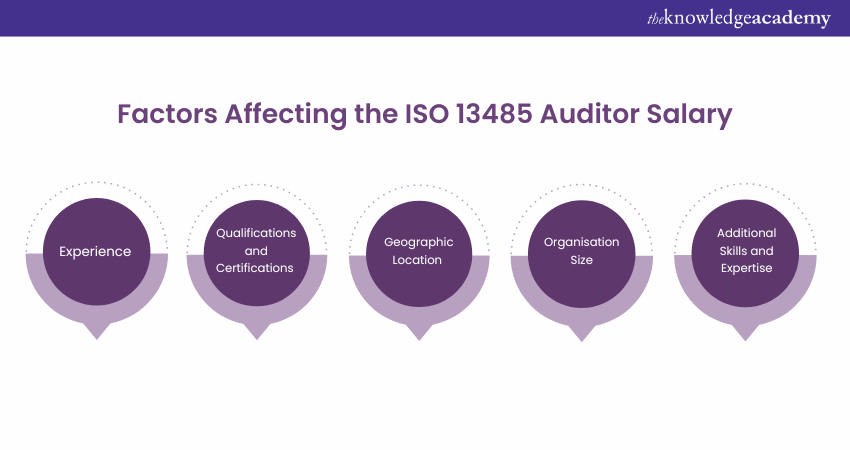
Experience
Experience plays a crucial role in highlighting an ISO 13485 Auditor's salary. As you gain more experience in the field, your value increases, and you can command higher compensation. Experienced auditors bring a wealth of knowledge, industry insights, and a track record of successful audits, which makes them highly sought after.
Qualifications and Certifications
Your qualifications and certifications significantly impact your earning potential as an ISO 13485 auditor. Holding relevant certifications, such as the ISO 13485 Lead Auditor Certification or the Certified Quality Auditor (CQA) designation, demonstrates your expertise and commitment to professional development. These certifications enhance your credibility and can positively influence your salary negotiations.
Geographic Location
Your location can influence your salary range as an ISO 13485 Auditor. Salaries may vary based on regional economic factors, cost of living, and market demand for Auditors in specific locations. Metropolitan areas and regions with a better concentration of medical device companies may offer more competitive salaries.
Organisation Size
The size of your organisation can impact your salary as an ISO 13485 Auditor. Large organisations often have more complex quality management systems and higher stakes in compliance. Consequently, they may offer higher salaries to attract experienced auditors who can effectively assess their systems. Smaller organisations may have different budget constraints, resulting in a lower salary range.
Additional Skills and Expertise
It can positively impact your salary range if you possess additional skills and expertise beyond ISO 13485 auditing, such as knowledge of other quality management systems or specialised knowledge within the medical device industry. These additional competencies make you an asset to organisations seeking comprehensive auditing capabilities.
Position your organisation as a leader in quality and compliance – join our ISO 13485 Training now!
Conclusion
By assessing compliance with the ISO 13485 standard, Auditors play an important role in ensuring the medical device industry's highest quality, safety, and regulatory compliance standards. We hope this blog has helped you learn about the ISO 13485 Auditor Salary and the factors that affect it.
Take the next step in your career as an ISO 13485 auditor with our ISO 13485 Lead Auditor Course today!
Frequently Asked Questions

Experience significantly impacts the salary of an ISO 13485 Auditor. Entry-level Auditors earn between £25,000 and £35,000 annually, while those with more experience can earn up to £50,000 or more.

ISO 13485 Auditors have strong career growth prospects, including advancing to Senior Auditor roles, specialising in specific areas, or moving into leadership positions.

The Knowledge Academy takes global learning to new heights, offering over 30,000 online courses across 490+ locations in 220 countries. This expansive reach ensures accessibility and convenience for learners worldwide.
Alongside our diverse Online Course Catalogue, encompassing 19 major categories, we go the extra mile by providing a plethora of free educational Online Resources like News updates, Blogs, videos, webinars, and interview questions. Tailoring learning experiences further, professionals can maximise value with customisable Course Bundles of TKA.

The Knowledge Academy’s Knowledge Pass, a prepaid voucher, adds another layer of flexibility, allowing course bookings over a 12-month period. Join us on a journey where education knows no bounds.

The Knowledge Academy offers various ISO 13485 Training, including the ISO 13485 Foundation Course. ISO 13485 Lead Auditor Course, and ISO 13485 Lead Implementer Course. These courses cater to different skill levels, providing comprehensive insights into ISO 13485.
Our ISO & Compliance Blogs cover a range of topics related to ISO 13485 Framework, offering valuable resources, best practices, and industry insights. Whether you are a beginner or looking to advance your Compliance skills, The Knowledge Academy's diverse courses and informative blogs have got you covered.
Upcoming Health & Safety Resources Batches & Dates
Date
 ISO 13485 Foundation
ISO 13485 Foundation
Mon 13th Jan 2025
Mon 28th Apr 2025
Mon 11th Aug 2025
Mon 10th Nov 2025







 Top Rated Course
Top Rated Course



 If you wish to make any changes to your course, please
If you wish to make any changes to your course, please


