We may not have the course you’re looking for. If you enquire or give us a call on +34 932716793 and speak to our training experts, we may still be able to help with your training requirements.
Training Outcomes Within Your Budget!
We ensure quality, budget-alignment, and timely delivery by our expert instructors.

ISO 17025 Implementation involves establishing and maintaining Quality Management systems in testing and calibration labs. The process includes gap analysis to identify areas for improvement, documenting policies and procedures, providing training to enhance staff competence, and ensuring adequate equipment and facilities. This systematic approach ensures that the labs operate competently, generate reliable results, and increase confidence in their services.
According to ISO, over 80,000 laboratories across various industries have been accredited to ISO 17025. Further, read this blog on ISO 17025 Implementation to uncover the key steps to follow for a smooth and successful adoption of this internationally recognised laboratory standard. Continue reading to learn more!
Table of Contents
1) What is ISO 17025 Implementation?
2) ISO 17025 Implementation process
3) Gap analysis with ISO 17025 Implementation
4) Internal audits and management review
5) Benefits of ISO 17025 Implementation
6) Conclusion
What is ISO 17025 Implementation?
ISO 17025 Implementation is the process of adopting and adhering to the requirements outlined in the ISO 17025 Standard for testing and calibration labs. It involves establishing a systematic approach to ensure that labs operate competently and generate reliable results. During implementation, labs evaluate their existing practices, procedures, and equipment to identify areas for improvement.
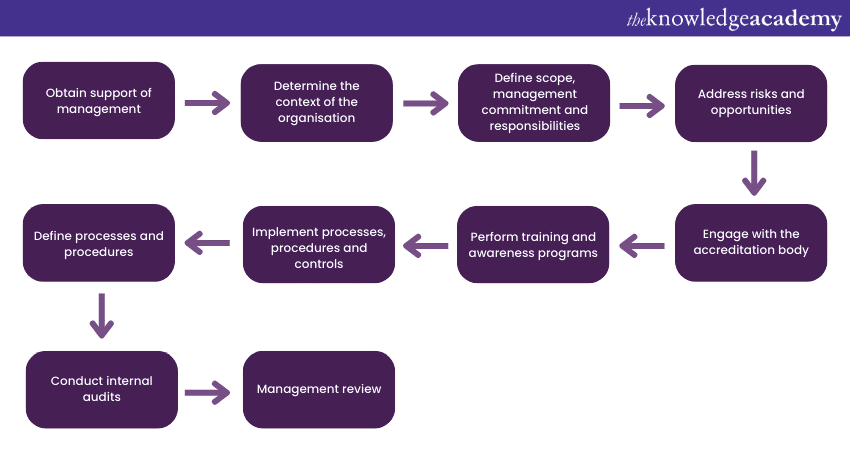
They develop and document quality policies, procedures, and work instructions to guide their operations. Staff receive training to enhance their skills and knowledge. One unique aspect of ISO 17025 Implementation is the focus on continual improvement. ISO 17025 Implementation also enables labs to seek accreditation. Accreditation involves an external evaluation, affirming that the lab meets ISO 17025 requirements. It adds credibility, trust, and international recognition.

ISO 17025 Implementation process
When implementing ISO 17025 in a laboratory, several key steps are involved that includes:
a) Initial audit: Conduct an assessment of the laboratory's practices and procedures against the Polish Centre for Accreditation (PCA) requirements and the PN-EN ISO 17025:2018 Standard. This audit helps identify areas that need improvement to meet the standard's criteria.
b) Developing documents: Creating the necessary documentation following the PN-EN ISO 17025:2018 Standard. This includes quality policies, procedures, and work instructions that outline how the laboratory operates and ensures compliance with the standard.
c) Training: Providing training to laboratory personnel on ISO 17025:2018, covering the basic requirements of the standard and the accreditation requirements set by PCA. This helps the staff understand the principles and guidelines they need to follow to maintain compliance.
d) Accreditation application: Assisting the laboratory in verifying and preparing their accreditation application to be submitted to the PCA. This involves ensuring that all necessary documentation and requirements are met to increase the likelihood of a successful application.
e) Accreditation process support: Offering support and guidance throughout the PCA accreditation process. This may involve answering questions, addressing concerns, and assisting in ensuring a smooth and successful accreditation journey.
Level up your auditing skills with our ISO 17025 Lead Auditor Training. Sign up now to enhance your expertise and become a certified Auditor!
Gap analysis with ISO 17025 Implementation
Gap analysis is a crucial step in ISO 17025 Implementation for labs. It involves comparing their current practices against the standard's requirements. Gaps or discrepancies are identified, such as inadequate documentation, training, or equipment calibration. During gap analysis, the lab compares its current practices, procedures, and resources against the specific criteria outlined in ISO 17025. It aims to find any "gaps" or discrepancies between the existing state of the lab and the desired state of compliance with the standard.
A team evaluates the lab's processes, documentation, and personnel competence to pinpoint areas that need improvement. Once gaps are identified, an action plan is created to address them and move closer to ISO 17025 compliance. This can involve updating procedures, enhancing training, or implementing quality control measures. Gap analysis provides labs with a roadmap for improvement, ensuring their services become more reliable, accurate, and credible. It helps them align with the standard's requirements and enhance their overall operations.
Master ISO 17025 Implementation with our ISO 17025 Lead Implementer Training now!
Internal audits and management review
Internal audits involve conducting periodic assessments within the laboratory to evaluate its compliance with ISO 17025 requirements. These audits are typically performed by trained staff independent from the audited areas. The purpose is to identify any non-compliance or areas for improvement. Various aspects of the laboratory's operations, including processes, documentation, equipment, and personnel competence, are examined during an internal audit.
Management review is a systematic evaluation conducted by laboratory management to assess the overall performance and effectiveness of the implemented Quality Management system. This review involves analysing data, including audit findings, customer feedback, and process performance indicators. The management review allows for a comprehensive assessment of the laboratory's compliance, identifying areas of success and areas that require attention.
Improve your auditing skills with our ISO 17025 Internal Auditor Training. Join now for certified competence!
Benefits of ISO 17025 Implementation
Implementing ISO 17025 in a lab offers several benefits, some of which include:
a) It provides credibility, thus enhancing the lab's reputation and assuring reliable results.
b) It sticks to quality improvement and ensures strict quality control for accurate testing.
c) Global Recognition allows global reach in international markets.
d) Provides efficiency by streamlining processes, reducing errors, and minimising risks.
e) It offers a commitment to staying updated with industry practices.
f) It includes competence development improving staff expertise and reliability.
g) It delivers consistent, high-quality results, building trust and leading to customer satisfaction.
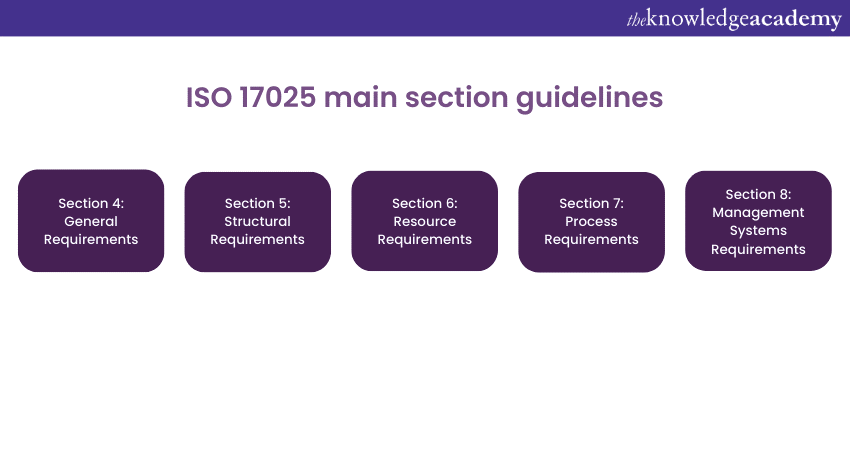
Conclusion
Effective ISO 17025 Implementation in a laboratory ensures credibility, quality, and customer satisfaction. It enhances reputation, provides global recognition, and improves efficiency. Embracing ISO 17025 demonstrates a commitment to quality, builds customer confidence, and sets labs apart in the competitive landscape.
Deepen your knowledge of ISO 17025's key terms and normative references with our ISO 17025 Training!
Frequently Asked Questions
Upcoming Health & Safety Resources Batches & Dates
Date
 ISO 17025 Foundation
ISO 17025 Foundation
Mon 27th Jan 2025
Mon 31st Mar 2025
Mon 18th Aug 2025
Mon 24th Nov 2025







 Top Rated Course
Top Rated Course


 If you wish to make any changes to your course, please
If you wish to make any changes to your course, please


