We may not have the course you’re looking for. If you enquire or give us a call on +30 2111995372 and speak to our training experts, we may still be able to help with your training requirements.
Training Outcomes Within Your Budget!
We ensure quality, budget-alignment, and timely delivery by our expert instructors.

If you’re involved in the world of testing and calibration laboratories, you’ve likely to come across the term ISO 17025. But what does it imply, and why are ISO 17025 Principles crucial?
The ISO 17025 Principles describe the essential guidelines and requirements for testing and calibration laboratories' competence, impartiality, and consistency. So, it is crucial to understand the Principles of ISO 17025 to ensure that laboratories operate with the highest quality, reliability, and integrity.
Still confused about the 17025 Principles? You need not juggle through the web anymore. Read this blog to gain in-depth understanding of ISO 17025 Principles. Also, learn the principles for a successful and reliable laboratory operation.
Table of Contents
1) Understanding the Importance of ISO 17025 Principles
2) What are the various Principles of ISO 17025?
a) Capacity
b) Exercise of Responsibility
c) Scientific Method
d) Objectivity of Results
e) Impartiality of Conduct
f) Traceability of Measurement
g) Repeatability of Test
h) Transparency of Process
3) Conclusion
Understanding the Importance of ISO 17025 Principles
The principles of ISO 17025 serve as the backbone for laboratory operations. It holds significant importance in ensuring the integrity and reliability of testing and calibration activities.
One of the key benefits of complying with these principles is the assurance of accurate and reliable results. Laboratories that follow these principles meticulously have robust quality management systems that encompass defined processes, thorough documentation, and systematic reviews.
This systematic approach minimises the risk of errors, enhances the consistency of results, and ensures the reliability of laboratory outcomes.

What are the various Principles of ISO 17025?
ISO 17025 is the international standard developed for testing and calibration laboratories built on fundamental principles that guide laboratory operations. Let’s explore the various principles that form the foundation of ISO 17025:
1. Capacity
Capacity refers to the laboratory’s ability to perform testing and calibration activities effectively and efficiently. It goes beyond having physical resources and includes the availability of skilled personnel, suitable equipment, and appropriate facilities to meet the demands of clients and deliver accurate results. By maintaining a strong capacity, laboratories can establish themselves as trusted partners in their respective industries.
2. Exercise of Responsibility
This principle in ISO 17025 underscores the crucial role laboratories play in ensuring the validity and accuracy of their testing and calibration activities. It entails a diligent and accountable approach to laboratory operations, emphasising the need for careful adherence to established processes and procedures.
Laboratories exercise responsibility by taking ownership of their work and striving for excellence in every aspect of their operations. This principle encompasses several key aspects, including:
a) Compliance with procedures
b) Quality management system
c) Employing competent and trained personnel
d) Equipment maintenance and calibration
e) Non-conformance management
f) Ethical conduct
3. Scientific Method
The Scientific Method is an organised and objective approach used in ISO 17025 to ensure the accuracy and reliability of testing and calibration. It involves the following:
a) Making observations and questioning
b) Formulating hypotheses and literature review
c) Conducting experiments
d) Analysing data
e) Drawing conclusions based on evidence
By following the Scientific Method, laboratories can ensure the rigorous and consistent application of scientific principles in their operations.
4. Objectivity of Results
The Objectivity of Results is another crucial principle in ISO 17025. It emphasises the need for impartiality and freedom from generation and interpretation biases. It also ensures that the reported data accurately reflects the characteristics of the sample being tested without any subjective influence or personal preferences. To uphold the Objectivity of Results, laboratories adhere to the following practices:
a) Employ standardised test methods and calibration procedures
b) Eliminate subjective influences
c) Perform blind testing
d) Implement quality control measures
e) Review and verify the results
This commitment to objectivity enhances the trustworthiness and credibility of the laboratory’s work in the eyes of its clients and stakeholders.
Learn the basic terminologies and definitions of ISO 17025 with our ISO 17025 Training.
5. Impartiality of Conduct
Impartiality emphasises the need for laboratories to act in a neutral and unbiased manner throughout their testing and calibration activities. It ensures that the laboratory's decisions, actions, and judgments are free from conflicts of interest and external influences, allowing for fair and objective results. Impartiality of conduct is maintained through the following practices:
a) Avoidance of conflicts of interest
b) Independence in decision-making
c) Treat all clients and samples equally
d) Maintain transparency
e) Uphold an ethical conduct
6. Traceability of Measurement
Traceability is the ability to link measurement results to national or international standards via an unbroken chain of comparisons. It involves establishing a clear and documented measurement chain, connecting the laboratory’s measurement results to internationally recognised standards. By maintaining traceability, laboratories ensure that their measurements are consistent, comparable, and traceable to a known reference.
7. Repeatability of Test
In ISO 17025, Repeatability of Test focuses on the consistency and precision of test results when the same procedure is repeated under similar conditions. It ensures that the measurement or testing process produces reliable and consistent outcomes, allowing for the replication of results within an acceptable margin of error. Here are key points to keep in mind regarding the Repeatability of the test:
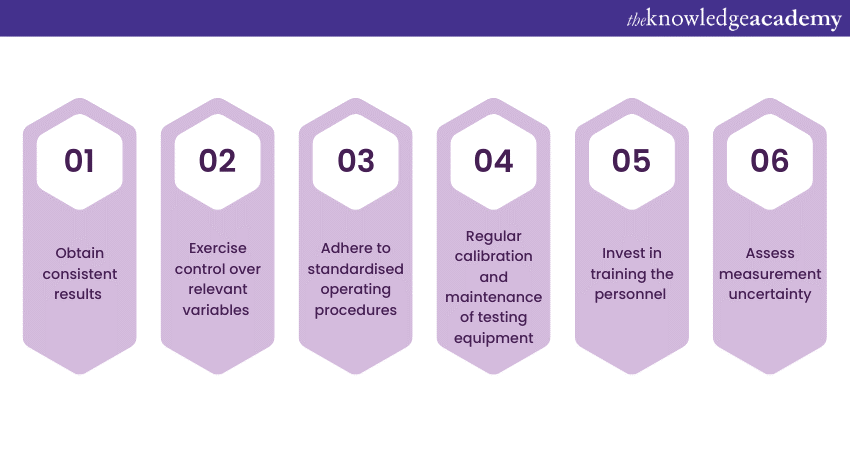
Transparency of Process
Transparency of Process emphasises the importance of openness and clarity in the operations and procedures of a laboratory. It provides stakeholders, including clients and regulatory bodies, with comprehensive and accessible information about the laboratory’s processes, methodologies, and quality management systems. Here are the key aspects involved in the Transparency of Process:
a) Clear communication
b) Document methods and procedures
c) Ensure quality and reliability
d) Provide accreditation and certifications
e) Generate detailed reports
f) Facilitating external audits and inspections
Learn how to create Audit checklists and Audit reports with our ISO 17025 Lead Auditor course.
Conclusion
The ISO 17025 Principles form the foundation for a robust quality management system. Laboratories that embrace these principles can demonstrate their commitment to excellence, continuous improvement, and adherence to international standards.
Enhance your skills in laboratory quality management. Register for our ISO 17025 Foundation course now!
Frequently Asked Questions
Upcoming Health & Safety Resources Batches & Dates
Date
 ISO 17025 Foundation
ISO 17025 Foundation
Mon 27th Jan 2025
Mon 31st Mar 2025
Mon 18th Aug 2025
Mon 24th Nov 2025







 Top Rated Course
Top Rated Course


 If you wish to make any changes to your course, please
If you wish to make any changes to your course, please


