We may not have the course you’re looking for. If you enquire or give us a call on +353 12338944 and speak to our training experts, we may still be able to help with your training requirements.
Training Outcomes Within Your Budget!
We ensure quality, budget-alignment, and timely delivery by our expert instructors.

Whether you are starting from scratch or looking to ensure compliance with ISO 17025, look no further than ISO 17025 Documentation. The guidelines provided in the documentation facilitate the organisations to adhere to ISO 17025 requirements and establish an effective quality management system.
But what does the documentation include? Why is it necessary? Read this blog to learn about ISO 17025 Documentation. You will also learn how to develop documentation to achieve compliance effectively.
Table of Contents
1) What does ISO 17025 Documentation mean?
2) Understanding the importance of ISO 17025 Documentation
3) Key elements of ISO 17025 Documentation
4) How to develop ISO 17025 Documentation?
5) Conclusion
What does ISO 17025 Documentation mean?
ISO 17025 Documentation serves as the backbone of quality management systems employed by testing and calibrating laboratories worldwide. This documentation plays a crucial role in ensuring the accuracy, reliability, and impartiality of testing and calibration activities.
It encompasses a wide range of documents, including procedures, work instructions, records, and forms. These documents outline the processes, methodologies, and controls necessary for conducting tests, calibrations, and measurements in a standardised and reliable manner.
Further, the primary purpose of the documentation is to provide laboratories with a framework for achieving and demonstrating compliance with the standard’s requirements. This promotes uniformity in testing and calibration processes and enhances the reliability of outcomes.
It is worth noting that 17025 documentation is not a one-time work but a continuous process. Laboratories must regularly review, update, and improve their documentation to remain current, accurate, and aligned with changing requirements and best practices.

Understanding the importance of ISO 17025 Documentation
The 17025 Documentation holds immense significance in testing and calibration laboratories. It serves as the foundation for establishing and maintaining an effective quality management system that adheres to international standards. Let’s delve into the key reasons why this documentation is crucial for your laboratory:
a) Ensuring consistency and standardisation: By documenting procedures, work instructions, and forms, you create a consistent approach to how activities are carried out. This ensures that all personnel involved in testing and calibration follow standardised protocols, minimising errors and variations in results.
b) Demonstrating compliance with ISO 17025: Compliance with ISO 17025 is vital for laboratories seeking to enhance their credibility and gain recognition. Well-documented processes and records act as tangible evidence for compliance with the standard's requirements.
c) Enhancing accuracy and reliability: By documenting equipment requirements, test methods, and acceptance criteria, laboratories can ensure that testing processes are performed consistently.
d) Facilitating traceability and accountability: Documentation of calibration procedures, equipment maintenance records, and sample handling processes enables laboratories to establish a clear traceability chain. This enhances the credibility of test results and fosters accountability and transparency.
e) Supporting continuous improvement: Regular review and update of documentation facilitate a culture of continuous improvement and ensure the efficiency of the quality management system.
Gain an in-depth of the Audit concepts and principles. Register for our ISO 17025 Internal Auditor course.
Key elements of ISO 17025 Documentation
The 17025 Documentation encompasses various essential elements for establishing and maintaining a robust quality management system in testing and calibration laboratories. These elements provide the foundation for ensuring compliance with the standard’s requirements and facilitate accurate and reliable testing and calibration activities. Let’s explore the key elements of 17025 Documentation:
Quality manual
The quality manual is the central document outlining the laboratory's quality management system. It provides an overview of the laboratory's organisational structure, key processes, and procedures.
This document also describes how the laboratory meets the requirements of ISO 17025. In simple terms, a quality manual helps set the tone for the entire documentation system.
Procedures
Procedures document the step-by-step instructions for performing specific processes within the laboratory. These processes include sample handling, equipment calibration, method validation, internal audits, and corrective actions.
This document provides consistency, standardisation, and clarity to ensure that activities are carried out in a controlled and reliable manner.
Work instructions
It is a detailed guide on performing specific tasks within a process. Work instructions include specific information such as equipment settings, safety precautions, sample preparation, data recording, and data interpretation. These are essential for ensuring the uniformity and accuracy of testing and calibration procedures.
Records
Evidence of activities performed within the laboratory form records. They include test reports, calibration certificates, equipment maintenance logs, training records, and audit findings. Records demonstrate the laboratory’s compliance with ISO 17025 requirements, provide traceability, and support data integrity.
Enhance your compliance skills to ensure the highest standards in your laboratory with our ISO 17025 Training.
Forms and templates
Forms and templates are standardised documents that facilitate the collection and recording of data. They provide a consistent format for capturing essential information about sample identification, test results, calibration parameters, and other data. Therefore, they ensure data is recorded accurately and consistently across different processes and personnel.
Management of change
ISO 17025 requires laboratories to document any changes made to procedures, equipment, or processes that may impact the quality of results. Therefore, laboratories develop the management of change documents.
Processes like identifying changes, evaluating their potential impact, updating relevant documentation, and communicating changes to personnel are noted in the document. This ensures that modifications are properly controlled and documented to maintain the integrity of the quality management system.
Training and competence records
ISO 17025 also emphasises the importance of personnel competence in performing testing and calibration activities. Documentation related to training records, qualifications, and competence assessment ensures that personnel possess the necessary skills and knowledge to carry out their assigned tasks effectively. As a result, it helps ensure that laboratory personnel are adequately trained and competent in their respective roles.
Internal audits
Internal audits involve the following:
a) Regular assessments of the laboratory’s quality management system to identify areas for improvement
b) Verify compliance with documented procedures
c) Ensure the effectiveness of controls.
These records demonstrate the laboratory’s commitment to continual improvement and provide valuable insights for enhancing the documentation system.
How to develop ISO 17025 Documentation?
Developing 17025 Documentation is a systematic process that involves careful planning, collaboration, and attention to detail. Here are the steps you need to follow to develop ISO 17025 Documentation:
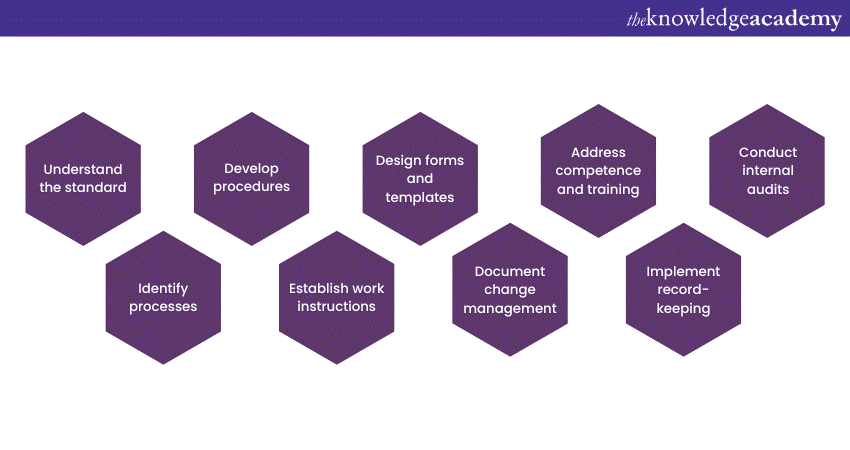
a) Familiarise yourself with the requirements of ISO 17025.
b) Identify your laboratory's key processes, including sample handling, equipment calibration, testing procedures, data analysis, and reporting, that require documentation.
c) Create step-by-step procedures, responsibilities, and required controls for each identified process.
d) Develop detailed work instructions to provide guidance on how to carry out activities, including equipment settings, safety precautions, and data recording.
e) Create standardised forms and templates to capture relevant data during testing and calibration activities.
f) Establish a process to manage and document any changes to procedures, equipment, or processes that may affect the quality of results.
g) Document qualifications, training records, and competence assessments to ensure staff members possess the necessary skills and knowledge to perform their roles effectively.
h) Implement a robust system for record-keeping.
i) Establish an internal audit program to assess the effectiveness of your 17025 Documentation system.
Conclusion
Embark on this ISO 17025 Documentation development journey and pave the way for excellence in your laboratory’s quality management system. With a well-structured and comprehensive documentation system in place, you can confidently meet stakeholders’ expectations, gain recognition, and continually improve your laboratory’s performance.
Lay a strong foundation for laboratory excellence with our ISO 17025 Foundation course.







 Top Rated Course
Top Rated Course



 If you wish to make any changes to your course, please
If you wish to make any changes to your course, please


