We may not have the course you’re looking for. If you enquire or give us a call on +44 1344 203 999 and speak to our training experts, we may still be able to help with your training requirements.
Training Outcomes Within Your Budget!
We ensure quality, budget-alignment, and timely delivery by our expert instructors.
If you have worked in testing and calibration laboratories, you must know about ISO 17025 accreditation. To ensure the quality and competence of laboratories, ISO provides detailed guidelines for providing several services. But knowing about the services covered under ISO 17025 Scope is crucial before getting accredited.
The ISO 17025 Scope defines the activities a laboratory can perform and the services it can provide. But what is covered under the Scope of ISO 17025? What services can the laboratories define as standardised? Don't worry more. We have brought this blog to help you find answers to these questions and more.
Furthermore, read this blog to discover the salient features of the ISO 17025 Scope. Also, learn about its significance, components, and implications across the industry.
Table of contents
1) What is ISO 17025?
2) Understanding ISO 17025 Scope
3) Components of a scope statement
4) What are the implications of ISO 17025 Scope?
5) Conclusion
What is ISO 17025?
ISO 17025 is an international standard developed by the International Organisation for Standardisation (ISO) and the International Electrotechnical Commission (IEC). It serves as a benchmark for laboratories involved in testing and calibration activities, ensuring their technical competence, impartiality, and reliability.
ISO 17025 outlines the requirements for establishing and maintaining a quality management system within a laboratory. It covers various aspects, including personnel competence, equipment calibration, testing methodologies, quality assurance, and the handling of test items.
This standard applies to various laboratories in manufacturing, healthcare, environmental analysis, and research sectors. It provides a framework for laboratories to demonstrate their ability to produce accurate and reliable results, fostering confidence in their services.
As a result, it helps laboratories enhance their credibility, meet customer expectations, and ensure compliance with relevant industry regulations. It also facilitates international acceptance and recognition of testing and calibration results, enabling effective collaboration and global market access.

Understanding ISO 17025 Scope
ISO 17025 accreditation provides laboratories with a mark of quality and competence in their testing and calibration activities. A fundamental aspect of ISO 17025 is the scope. The ISO 17025 Scope of the accreditation refers to the specific activities and services a laboratory can perform.
It outlines the types of tests and calibrations the laboratory can carry out, the methods employed, and the circumstances under which these activities are conducted. The scope statement should be concise, clear, and aligned with the laboratory’s actual capabilities.
Further, defining the scope is paramount for laboratories seeking ISO 17025 accreditation. It allows the laboratory to focus its efforts and resources on areas where it has demonstrated competence. The scope assures customers that the laboratory possesses the necessary expertise and facilities to carry out the required tests or calibrations effectively.
A well-defined scope ensures the laboratory’s activities are consistent with its demonstrated competence. It helps manage customer expectations, minimises misunderstandings, and builds confidence in the laboratory’s services.
Moreover, the scope statement acts as a marketing tool, showcasing the laboratory’s capabilities to potential customers. Therefore, it helps the laboratory stand out in the market by highlighting its areas of expertise and specialisation.
Understand how to create Audit checklists and Audit reports with our ISO 17025 Lead Auditor course.
Components of a scope statement
The scope statement is crucial to ISO 17025 accreditation for testing and calibration laboratories. A well-defined scope statement is essential for effective communication, setting accurate expectations, and maintaining the integrity of the accreditation. It typically includes the following components:
a) Identification of the laboratory: The scope statement should begin by clearly identifying the laboratory for which it is intended. This includes the laboratory’s name, location, and any other relevant identifying information.
b) Description of the laboratory’s activities: It should specify the types of tests and calibrations that the laboratory is accredited to perform. Further, the scope statement should also outline the specific areas and disciplines the laboratory specialises in.
c) Specification of the methods and procedures used: The statement should provide methods, procedures, and techniques employed by the laboratory to conduct the accredited tests and calibrations. This helps ensure consistency and reliability in the laboratory’s processes.
d) Reference to relevant standards and regulations: It should also mention the applicable standards and regulations the laboratory adheres to in its accredited activities. This highlights the laboratory’s commitment to quality and compliance.
e) Identification of any exclusions or limitations: If there are any specific tests, calibrations, or areas that the laboratory excludes from its scope, these exclusions should be clearly stated in the scope. This ensures transparency and avoids any misunderstandings regarding the laboratory’s capabilities.
Grab the chance to improve your laboratory's credibility. Register for our ISO 17025 Training now!
What are the implications of ISO 17025 Scope?
The scope defined within ISO 17025 accreditation holds significant implications for testing and calibration laboratories. It not only influences the laboratory's operations but also impacts its credibility, compliance, and customer satisfaction.
Understanding the implications of the Scope of ISO 17025 is crucial for laboratories aiming to maintain their accreditation and meet industry requirements. Here are some key implications to consider:
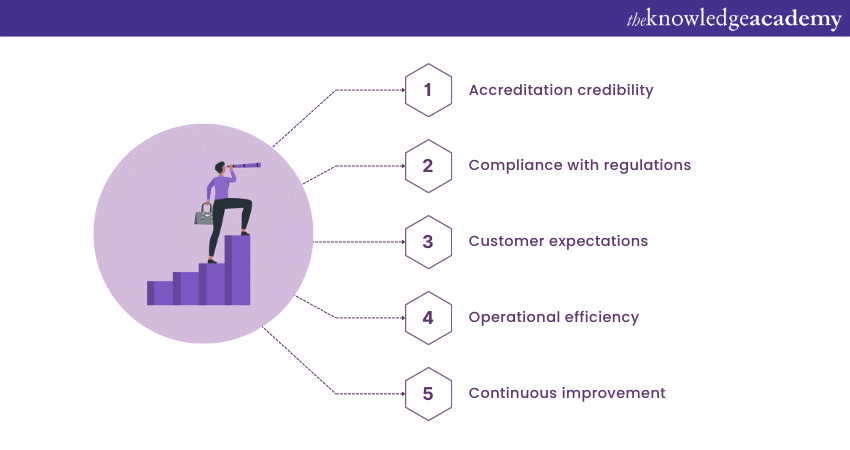
a) Accreditation credibility: The scope of accreditation demonstrates the laboratory’s competence in specific tests or calibrations, giving customers confidence in the reliability of its results. A well-defined scope enhances the laboratory’s reputation and fosters trust among stakeholders.
b) Compliance with regulations: The scope ensures that the laboratory complies with relevant regulations and standards. The laboratory can demonstrate its commitment to quality and regulatory compliance by doing so. As a result, it can even avoid potential legal issues and maintain high professionalism.
c) Customer expectations: The scope statement helps customers understand the types of tests or calibrations that the laboratory can perform. It also states any limitations that may exist. Accurate communication of the scope allows customers to make informed decisions about using the laboratory’s services and prevents misunderstandings.
d) Operational efficiency: By aligning their operations with the scope, laboratories can optimise their processes, enhance efficiency, and allocate resources effectively. This leads to improved productivity and cost-effectiveness in delivering testing and calibration services.
e) Continuous improvement: The scope of ISO 17025 accreditation provides a framework for laboratories to improve their operations continuously. It encourages laboratories to review, expand their scope, and incorporate new tests, calibrations, or technologies to enhance their capabilities.
Conclusion
Understanding the ISO 17025 Scope is crucial for defining the boundaries and extent of a laboratory’s accredited activities. It provides clarity to both the laboratory and its customers, ensuring they have the right expectations and reliable results. A well-crafted scope statement enhances the laboratory’s credibility, compliance with regulations, and customer satisfaction.
Learn about the principles of ISO 17025 for testing and calibration laboratories with our ISO 17025 Foundation course.







 Top Rated Course
Top Rated Course



 If you wish to make any changes to your course, please
If you wish to make any changes to your course, please


