We may not have the course you’re looking for. If you enquire or give us a call on 01344 203999 and speak to our training experts, we may still be able to help with your training requirements.
Training Outcomes Within Your Budget!
We ensure quality, budget-alignment, and timely delivery by our expert instructors.

In the highly regulated medical industry, ISO 13485 Compliance is essential for any organisation producing medical devices. The standard plays a critical role in safeguarding the safety and efficiency of medical devices. Following such international quality standards can have many benefits.
Getting this certificate helps ensure compliance with the highest Quality Management standards, following the regulations, and customer satisfaction. This blog provides a detailed explanation of ISO 13485 Compliance, highlighting and implementing the standards within an organisation. Read more!
Table of Contents
1) How to comply with ISO 13485?
2) Steps for ISO 13485 Certification
3) Best practices for maintaining ISO 13485 Compliance
4) Conclusion
How to comply with ISO 13485?
ISO 13485 represents the most commonly adopted international standard for Quality Management in the medical device sector. Published by the International Organization for Standardization (ISO), this standard offers a robust framework to fulfil the extensive Quality Management System (QMS) needs specific to the medical device industry. Now, such a framework makes ISO 13485 the foremost ISO standard in the domain of medical devices.
To establish a QMS, it is essential to receive support from management and determine the client's requirements. Creating vital documents such as the Quality Policy, Quality Objectives, and Quality Manual is the first step in this process. These documents collectively define the scope and application of the Quality Management System. It is also crucial to develop both mandatory and supplementary processes and procedures that are necessary for your organization to produce and deliver your product or service efficiently.
Quality Management System (Clause 4)
The ‘Intersection’ Clause 4 of a Quality Management System focuses on two main areas, namely General Requirements and Documentation Requirements. General Requirements emphasise systematic necessities for setting up and running a Quality Management System as per ISO standards, particularly ISO 13485. These include:
a) Compliance with the standard
b) Gathering the necessary documentation
c) Sustaining the required standards
d) Establishing and validating the effectiveness of written procedures
e) Assessing and managing risks in all operations
f) Implementing the necessary measures to mitigate identified risks and prevent severe incidents
g) Establishing and adhering to procedures for medical device production
h) Monitoring activities, rectifying any process errors, and maintaining records to demonstrate compliance
i) Abiding by legal obligations
j) Retaining responsibility for outsourced work
k) Verifying the efficiency of manufacturing systems and their impact on processes
More importantly, documentation requirements stress the importance of a Quality Manual as a fundamental element within QMS. Organisations must commit to fostering a quality-centric environment and culture. Such a commitment is often reflected in a policy or objective statement. The standard outlines explicit requirements for both procedures and records:
a) The maintaining of a file for medical device production with detailed product information and usage instructions.
b) Devising strategies for document control.
c) Strategies for record control
Management responsibility (Clause 5)
Management should exhibit their dedication by accepting responsibility for their organisation's activities. Moreover, it is crucial for them to maintain attention on the end user's requirements and adhere to legal standards in production. Now leaders are fundamentally obligated to uphold the quality policy, ensure that it complies with local legislation, and convey the company's objectives to their team. Their duties typically include strategic planning, assigning roles, and clear communication. Additionally, they are accountable for regularly examining and enhancing the organisation's processes, a task referred to as the ‘Management Review’.
Resource management (Clause 6)
Senior management is tasked with guaranteeing that the Quality Management System aligns with ISO 13485 standards and complies with local regulatory norms. Additionally, as per ISO 13485, it is imperative for top-level executives to provide sufficient resources for fulfilling the organisation's commitments. Resource provision encompasses staffing, infrastructure, consumables, equipment, planning for future leadership, and minimising risks. Such responsibilities range from overseeing daily operations to preventing contamination, and ensuring smooth future operations with proactive planning for impending retirements. This commitment from management, while seemingly modest, is important for the success of the organisation in the field of medical device manufacturing, as stipulated in Clause 6.
Product realisation (Clause 7)
An organisation needs to carefully navigate the path from idea to execution. The navigation process involves the establishment of a method for recording the initiation of ideas, validation of concepts, and the design and development of products, along with processes for their verification and validation, to meet the standards of ISO 13485, Clause 7.
Now effective communication is essential in the design and development phase of the device. The process must be meticulously followed, starting with planning, then moving through inputs and outputs, to review, verification, and finally, validation. Further, the translation of ideas into actionable plans, managing design processes, documenting any changes, and maintaining all relevant records are crucial steps in product realisation. Moreover, the definition and refinement and monitoring supply chains, keeping detailed records for each product, and establishing verification procedures should be explicitly outlined.
Monitoring each stage of the process is crucial, including the maintenance of cleanliness, overseeing installation, performing necessary services, and adhering to specific medical device requirements. Efficient management and maintenance of equipment, ensuring proper identification of devices, and components of product realisation are essential. Lastly, tracking the product's effectiveness, managing customer property, and ensuring product preservation are critical for achieving compliance with ISO 13485.
Measurement, analysis, and improvement (Clause 8)
Once your product is manufactured and available for public use, you bear the responsibility to ensure it satisfies your consumers’ needs. Now the key to achieving this is by gathering feedback. As outlined in Clause 8, the creation of a process for efficiently monitoring and measuring the success of the product should encompass:
a) Addressing all customer complaints,
b) Reporting any incidents to regulatory bodies,
c) Conducting internal audits for self-assessment,
d) Continuously evaluating both the process and the product internally,
e) Identifying and managing products that fail to meet initial design specifications (nonconforming products),
Analysing the collected data to constantly enhance the process.
Unlock career growth with ISO 13485 Training – Dive into quality management expertise!
Steps for ISO 13485 Certification
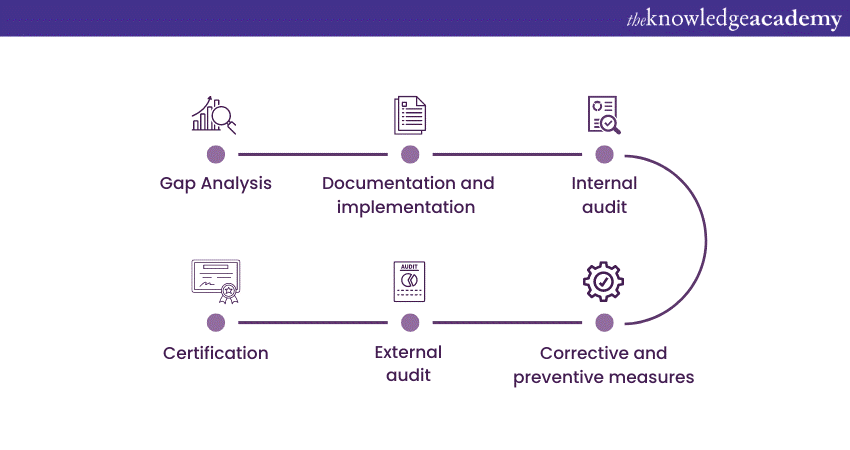
Use the following steps to get ISO 13485 Certificate:
a) Gap analysis: Conduct a thorough assessment of your organisation's current QMS against the ISO 13485 Requirements to identify areas that need improvement.
b) Documentation development: Develop and document the necessary policies, procedures, and work instructions to align with ISO 13485.
c) Implementation: Implement the Quality Management System, ensuring that all relevant personnel are trained and aware of their roles and responsibilities.
d) Internal Audit: Perform regular internal audits to evaluate whether the implemented QMS is effective. This also entails checking for any non-compliance.
e) Corrective and preventive actions: Take corrective and preventive actions to address identified non-conformities and improve the Quality Management System.
f) Certification body selection: Choose an accredited certification body to perform the certification audit.
g) Certification audit: The certification body (not ISO) will conduct an initial audit to assess the organisation's compliance with ISO 13485 requirements.
h) Corrective actions and Final audit: Address any non-conformities identified during the initial audit and undergo a final audit to verify compliance.
Certification: Upon completing the final audit, the certification body will issue ISO 13485 certification.
Following these steps will get you the certification, helping you comply with ISO 13485. This certification showcases your commitment to quality and compliance within the medical industry
Build a strong foundation in quality management with ISO 13485 Foundation Training – Start your learning journey today!
Best practices for maintaining ISO 13485 Compliance
Maintaining ISO 13485 Compliance is an ongoing commitment that requires continuous effort and vigilance. Here are some best practices to help organisations effectively sustain.
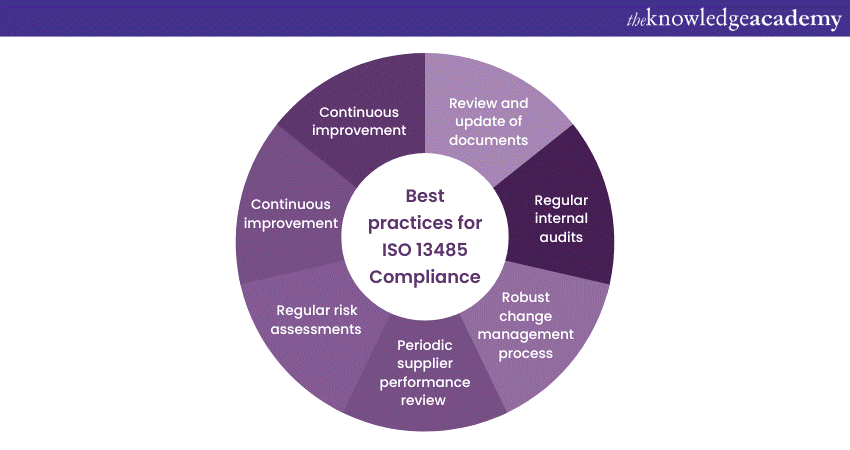
a) Review and update your documented procedures, work instructions, and forms to reflect process changes or regulatory requirements. Ensure that all relevant personnel can access the latest versions and know the document control procedures.
b) Perform regular internal audits to evaluate the effectiveness of your quality management system and identify areas for improvement. Perform thorough management reviews to evaluate the system's performance, set objectives, and identify opportunities for enhancement.
c) Implement a robust change management process to control and document any changes to processes, products, or systems. Assess the impact of changes on ISO 13485 Compliance and ensure that all changes are properly authorised, implemented, and communicated.
d) Periodically monitor and evaluate the performance of your suppliers to ensure their ongoing compliance with ISO 13485 requirements. Regularly review and update your supplier control processes to maintain effective supplier management.
e) Conduct periodic risk assessments to identify and evaluate potential risks associated with your medical devices and quality management system. Implement appropriate risk mitigation measures and review risk management activities to ensure effectiveness.
f) Promptly investigate and address any non-conformities, customer complaints, or incidents. Implement effective corrective and preventive actions to prevent recurrence and continuously improve your quality management system.
g) Continuous Improvement Culture: Foster a culture of continual improvement by encouraging employees to identify areas for enhancement and share ideas for process optimisation. Implement improvement projects and initiatives based on data-driven decision-making and customer feedback.
Staying up to date with changes in regulatory requirements and monitoring their impact on your organisation is of utmost importance. You must regularly review and update your quality management system to maintain compliance with relevant regulations.
Learn the basic principles and importance of ISO 13485 with our ISO 13485 Lead Implementer course.
Conclusion
Achieving ISO 13485 Compliance is essential for organisations in the medical device manufacturing industry. By adopting best practices, organisations can enhance product quality, mitigate risks, and gain a competitive advantage in the market. This certification brings numerous benefits, including improved processes, following the regulations, customer confidence, and global market access. Even though following the standard may seem daunting and requires effective document control, internal audits, training, and a commitment to continual improvement, but the benefits far outweigh the hassle.
Gain the knowledge and skills necessary to conduct internal audits of your organisation's QMS with our ISO 13485 Internal Auditor training.
Frequently Asked Questions

No, the ISO 13485 is not a compulsory standard, meaning you can develop a Quality Management System (QMS) tailored to your organisation's specific needs. However, this QMS must comply with the legal and regulatory requirements for medical devices in your target manufacturing and sales regions. Although not mandatory for EU MDR (Medical Device Regulation) compliance, the EU MDR does mandate having a QMS. Notably, ISO 13485:2016 is the sole QMS standard included in the EU's list of harmonised standards. Consequently, most companies opt to align their QMS with ISO 13485 requirements.

The ISO 13485 Compliance benefits your organisation in the following ways:
a) Boosting credibility: the ISO 13485 certification enhances company reputation by demonstrating commitment to high-quality standards.
b) Data-driven decisions: ISO 13485 enables a focus on quality objectives, offering continual data for strategic decision-making and addressing progress shortfalls.
c) Ongoing improvement: Adopting ISO 13485 instigates cultural shifts towards continual enhancement, streamlining processes, and fostering high performance.
d) Employee engagement: ISO 13485 clarifies employee roles, fostering involvement and insight for process improvement.
e) Customer satisfaction: Central to ISO 13485 is customer satisfaction, with certified companies perceived as more reliable,.

The Knowledge Academy’s Knowledge Pass, a prepaid voucher, adds another layer of flexibility, allowing course bookings over a 12-month period. Join us on a journey where education knows no bounds.

The Knowledge Academy takes global learning to new heights, offering over 30,000 online courses across 490+ locations in 220 countries. This expansive reach ensures accessibility and convenience for learners worldwide.
Alongside our diverse Online Course Catalogue, encompassing 17 major categories, we go the extra mile by providing a plethora of free educational Online Resources like News updates, Blogs, videos, webinars, and interview questions. Tailoring learning experiences further, professionals can maximise value with customisable Course Bundles of TKA.

The Knowledge Academy offers various ISO 13485 Training, including ISO 13485 Foundation, ISO 13485 Lead Auditor and many more. These courses cater to different skill levels, providing comprehensive insights into What is ISO 13485.
Our Health and Safety Blogs cover a range of topics related to ISO 13485, offering valuable resources, best practices, and industry insights. Whether you are a beginner or looking to advance your Compliance skills, The Knowledge Academy's diverse courses and informative blogs have you covered.
Upcoming Health & Safety Resources Batches & Dates
Date
 ISO 13485 Foundation
ISO 13485 Foundation
Mon 6th Jan 2025
Mon 3rd Mar 2025
Mon 5th May 2025
Mon 7th Jul 2025
Mon 8th Sep 2025
Mon 3rd Nov 2025







 Top Rated Course
Top Rated Course



 If you wish to make any changes to your course, please
If you wish to make any changes to your course, please


