We may not have the course you’re looking for. If you enquire or give us a call on +40 316317743 and speak to our training experts, we may still be able to help with your training requirements.
Training Outcomes Within Your Budget!
We ensure quality, budget-alignment, and timely delivery by our expert instructors.

Quality management is crucial for organisations in the medical device industry. To obtain this certification you must understand the ISO 13485 Audit Questions and Answers. It is a global benchmark for quality management systems, ensuring companies adhere to strict standards and regulations. However, getting this certification isn't very easy. It requires the organisation to meet strict requirements and pass an external audit.
Achieving this certification demonstrates a commitment to quality, safety, and compliance, instilling confidence in customers and regulatory authorities. This blog will give you insights into some of the most common ISO 13485 Audit Questions and Answers to help you get one step closer to the certification.
Table of Contents
1) Importance of ISO 13485 Certification
2) Who conducts the ISO 13485 auditing
3) Top 20 ISO 13485 Questions and Answers
a) What documentation is required for the ISO 13485 Certification?
b) What is the key difference between preventive and corrective actions?
c) What is the typical timeframe to obtain ISO 13485 Certification?
d) What is risk management in ISO 13495?
e) How should supplier controls be managed according to ISO 13485?
4) Conclusion
Importance of ISO 13485 Certification
ISO 13485 Certification validates that your organisation complies with the set international standards. This certification applies to anyone involved in the medical device domain at any stage, from design, manufacturing, distribution, and storage to implementation. Since medical device failures can have serious consequences and endanger a patient's life, all devices must comply with a practical quality standard.

Who conducts the ISO 13485 Auditing?
International Organization for Standardization (ISO) is the body that creates and maintains the quality standard, but it does not audit or certify the organisation. The certification and auditing process is carried out by third-party certified auditors who are experts in assessing the requirements or compliance with the ISO standard. Organisations can choose from various certification bodies accredited to perform ISO 13485 audits
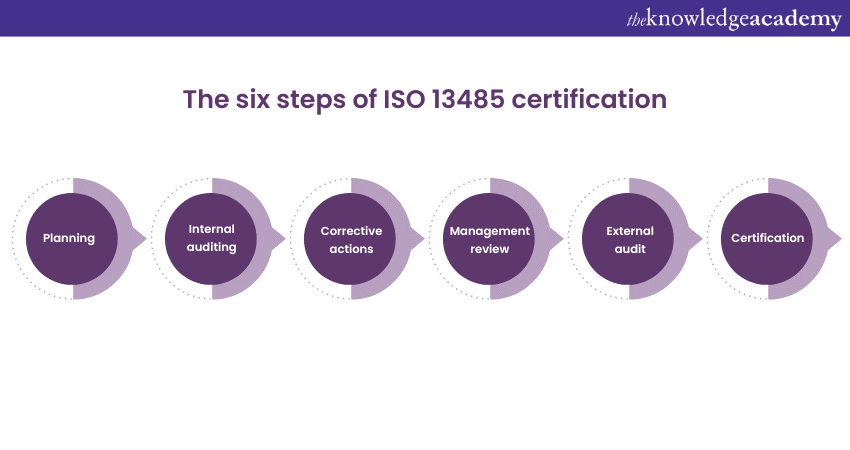
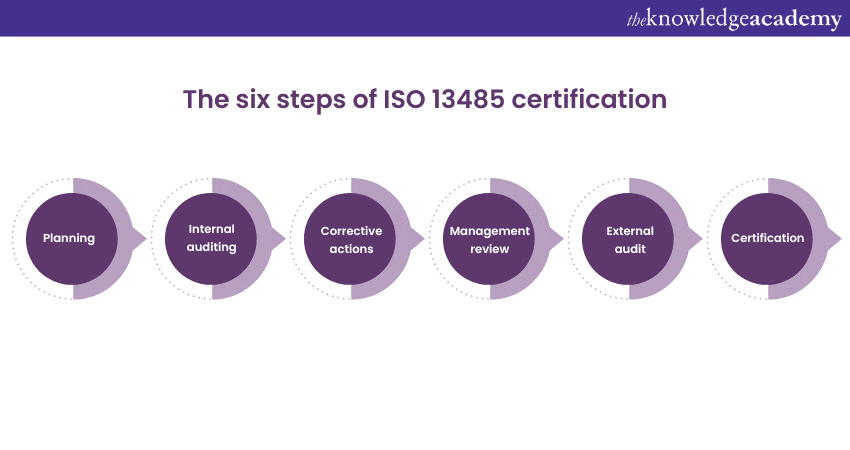
Internal auditing can help you determine if the medical devices manufactured by your organisation meet the quality standards. Carrying out such an audit will help you identify and correct any non-compliance or any other issue before the external audit.
Advance with ISO 13485 Training Courses – Elevate your expertise and lead in quality management!
Top 20 ISO 13485 Audit Questions and Answers
Here are some common questions asked during the ISO 13485 audit.
What documentation is required for the ISO 13485 Certification?
The essential documents required for the ISO 13485 Certification are the quality manual, procedures, work instructions, records, and policies relevant to the organisation's Quality Management System (QMS).
What is the key difference between preventive and corrective actions?
Corrective actions mitigate any non-compliance with the requirements. In contrast, preventive actions aim to identify and solve any potential issues or incidents that might occur. While they share the common goal of improving processes and preventing non-compliance, they address different aspects of quality management.
What is the typical timeframe to obtain ISO 13485 Certification?
The duration for getting the ISO 13485 Certification can vary depending on factors like the organisation's size, complexity, and readiness. It also depends on the preparedness of the organisation and the level of adherence to the standard. On average, obtaining the certification requires a time of three to six months. However, in some cases, getting the certificate might take longer.
What is risk management in ISO 13485?
Risk management is a systematic process that helps identify, evaluate and control the potential risks that could impact the performance and quality of medical devices. The primary goal of risk management is to understand the risks throughout the entire lifecycle of medical devices, from their design and manufacturing to usage. Following the latest revision in 2020, risk management has become a significant aspect of fulfilling the ISO 13485 Requirements.
Learn the fundamentals of ISO 134845 requirements and quality management system for medical devices with our ISO 13485 Foundation training.
How should supplier controls be managed according to ISO 13485?
Supplier controls are crucial in maintaining ISO 13485 compliance and ensuring that the quality and reliability of medical devices are maintained. It requires organisations to establish and maintain processes for selecting, evaluating, and monitoring suppliers. Major factors for assessing the supplier include supplier performance, experience and adherence to regulations.
A risk-based approach is recommended, and effective communication and collaboration are vital to managing supplier controls. Apart from that, supplier development and improvement are also encouraged.
What is the process for handling non-compliant products?
Handling non-compliant products is a crucial part of quality management within ISO 13485. It is recommended to establish clear processes for handling non-compliant products. The process of handling non-compliant products includes:
a) Identifying the non-compliant product via testing and analysing the customer feedback.
b) Next, it must be segregated and controlled to avoid its usage.
c) All the details regarding the non-compliance must be documented for traceability and proper record.
d) A detailed investigation must be carried out to identify the root cause, and based on the findings, preventive and corrective measures must be taken.
Can an organisation outsource activities under ISO 13485?
Yes, organisations are allowed to outsource certain activities. However, the organisation remains responsible for ensuring that the outsourced activities comply with the standard's requirements.
Learn how to conduct internal auditing and be prepared for the final external audit with our ISO 13485 Internal Auditor Training Course.
What is the key difference between validation and verification in ISO 13485?
In ISO 13485, verification and validation are two concepts that play an essential role in ensuring the quality and reliability of medical devices. While they are related processes, they serve different purposes within the framework.
Verification refers to evaluating and confirming that a product, system or component meets specific requirements. In comparison, validation focuses on ensuring the product or system meets the user's needs and intended use.
How does ISO 13485 address post-market surveillance?
ISO 13485 requires organisations to establish processes for monitoring and collecting post-market information. This includes complaints handling, vigilance reporting, and corrective actions related to product safety and performance.
What is the role of management in ISO 13485 implementation?
Top management is crucial in implementing ISO 13485 by demonstrating leadership, establishing quality standards and policies, ensuring that resources are available, and fostering a quality and continuous improvement culture.
How should organisations handle change control under ISO 13485?
Organisations must establish processes for controlling quality management system changes, including documents, processes, and medical devices. This ensures that changes are properly evaluated, authorised, and implemented.
Can organisations be certified to ISO 13485 without a quality manual?
According to ISO 13485, organisations don't need a quality certification manual. However, organisations must ensure that their QMS documentation adequately addresses the standard's requirements.
While a quality manual has traditionally been a common component of quality management systems, ISO 13485:2016 allows for much more flexibility in documentation requirements. The documentation should be comprehensive, controlled, and accessible to relevant personnel.
How are customer feedback and complaints addressed in ISO 13485?
Organisations should have proper processes for collecting, analysing, and responding to customer feedback and complaints. This helps identify the areas for improvement and ensures customer satisfaction and safety.
What is the purpose of the management review in ISO 13485?
The management review is a key component of ISO 13485. It involves top management evaluating the effectiveness and suitability of the quality management system, reviewing audit results, analysing data, and identifying opportunities for improvement.
Can ISO 13485 Certification be integrated with other management system standards?
Yes, ISO 13485 can be integrated with other standards like ISO 9001 (quality management) and ISO 14001 (environmental management). Integration can help streamline some processes and reduce duplicate efforts.
What is the role of training and competence in ISO 13485?
Personnel whose work affects product quality and compliance must be adequately trained. Necessary steps or actions must be taken to ensure that the personnel are competent enough to carry out the work and are trained to deliver quality work.
How often should organisations conduct management reviews?
It is recommended that management conduct reviews at planned intervals considering the organisation’s needs and the Importance of ISO 13485 quality management system. Typically, the management reviews are held annually or more frequently if needed.
Can ISO 13485 Certification be suspended or revoked?
Yes, ISO 13485 certification can be suspended or revoked if an organisation fails to meet the requirements or maintain its quality management system. Regular surveillance audits are conducted to monitor compliance.
Is ISO 13485 Certification mandatory for all medical device companies?
ISO 13485 Certification is not mandatory in all countries. However, it is widely recognised and often required by regulatory authorities and customers as evidence of a company's commitment to quality and compliance.
Learn and understand the benefits of implementing the ISO 13485 standard in your organisation. Join our ISO 13485 Lead Implementer training.
Conclusion
Obtaining ISO 13485 certification is crucial for organisations that are involved in the domain of medical devices. In this blog, we discussed the top commonly asked ISO 13485 Audit Questions and Answers that can help you get the certification. Adhering to its requirements and implementing effective quality management systems will enhance your reputation, promote regulatory compliance, and create a culture of continuous improvement that helps deliver safe and effective medical devices.
Gain an understanding of internal auditing to collect evidence and learn the roles and responsibilities of the lead auditor with the ISO 13485 Lead Auditor Training Course.
Frequently Asked Questions
Upcoming Health & Safety Resources Batches & Dates
Date
 ISO 13485 Foundation
ISO 13485 Foundation
Mon 13th Jan 2025
Mon 28th Apr 2025
Mon 11th Aug 2025
Mon 10th Nov 2025







 Top Rated Course
Top Rated Course


 If you wish to make any changes to your course, please
If you wish to make any changes to your course, please


